Your Cart is Empty
We have done a significant amount of evaluation to identify features of the optimal shRNA structure necessary to ensure our complex libraries maintain and express representative and effective shRNA. Independent from particular targeting sequences, structural variations such as defined mismatches, loop sizes, and stem lengths have significant affect on both the cloning and maintenance of the hairpin-coding insert in the heterogeneous library, as well as subsequent stability of the insert to integrate and stably express in the host cell. To provide comprehensive screening, it is critical to optimize the structure so that the library can maintain a representative copy number of all shRNA sequences with minimal bias through cloning, amplification, packaging, transduction, and selection.
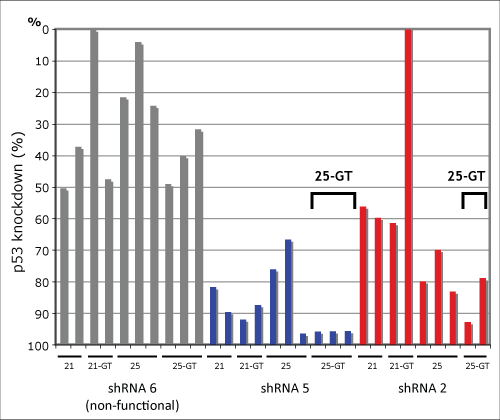
For the initial structural analysis, we made use of an shRNA-testing reporter construct using a destabilized green fluorescent protein (GFP). A description of this reporter system can be found in the Technology section of our website and more details will be available for publication shortly. I would just point out that, by combining this reporter construct with our library screening technology, we were able to analyze the effects of 40 different design variations of 150 different shRNA targeting sequences. Subsequent analysis by qRT-PCR of the best 10 designs showed significant variation as you can see from the data below that shows three examples of 10 variations of shRNA targeting the same region of the p53 gene. In the end, the best structure was a 25-base stem containing a few precise mismatches and a 7-nt loop.
One question we often get asked when presenting this optimization strategy is how the miRNA structure compares. In fact, we did not include miRNA variations in this study because we previously found that they anecdotally did not perform as well as shRNA. Cellular processing of miRNA to form the RNA-induced silencing complex requires digestion with both the Drosha and Dicer enzymes. Intuitively, the addition of the extra Drosha processing might be expected to make the effective concentration of the active siRNA form somewhat lower in the cells and also make it less universally effective across different cell lines since it depends on the effective activity of two enzymes instead of just the one. Anecdotally, we did find a lot of variation with miRNA forms that we tested. Published results from a CGAP-sponsored inhibition study of the shRNAmir miRNA variation also found similar results in terms of effectiveness and variation. The study contained a lot of interesting data presented many ways but the finding that particularly stood out what that, across more than 100 genes, only about 37% of expressed shRNAmir produced >75% knockdown in OVCAR-8 cells and just 12% provided 75% knockdown in MCF7 cells (See CGAP Study Figure 1). Hairpin shRNA structures routinely generated much better knockdown rates.